What we knew and what we were learning about vaccines and variants
The pandemic continued to evolve with the release of vaccines and as new variants emerged. People still needed to understand their own risks as both the virus and the response from the scientific and medical communities changed.
As more people were getting vaccinated, public health recommendations were being updated to reflect the new situation. As of May 13, 2021, the CDC’s official guidelines for the U.S. stated that fully vaccinated people no longer need to wear a mask or physically distance in most settings. These updates were a result of the high efficiency of the EUA COVID-19 vaccines and a growing body of evidence that fully vaccinated people were less likely to have an asymptomatic infection or transmit SARS-CoV-2 to others.20
As of April 2022, all EUA and FDA-approved COVID-19 vaccines being distributed were highly effective in preventing COVID-19. In international, randomized, placebo-controlled studies, the Pfizer-BioNTech and Moderna COVID-19 Vaccines were 95% and 94.1% effective in preventing COVID-19 disease.21,22 The Janssen vaccine was 77% effective in preventing severe/critical COVID-19 occurring at least 14 days after vaccination and 85% effective in preventing severe/critical COVID-19 occurring at least 28 days after vaccination.23
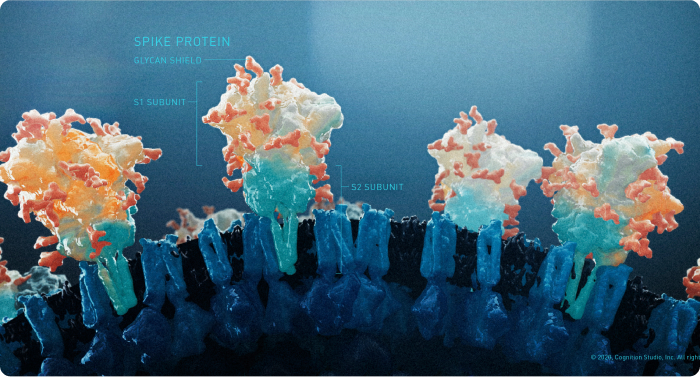
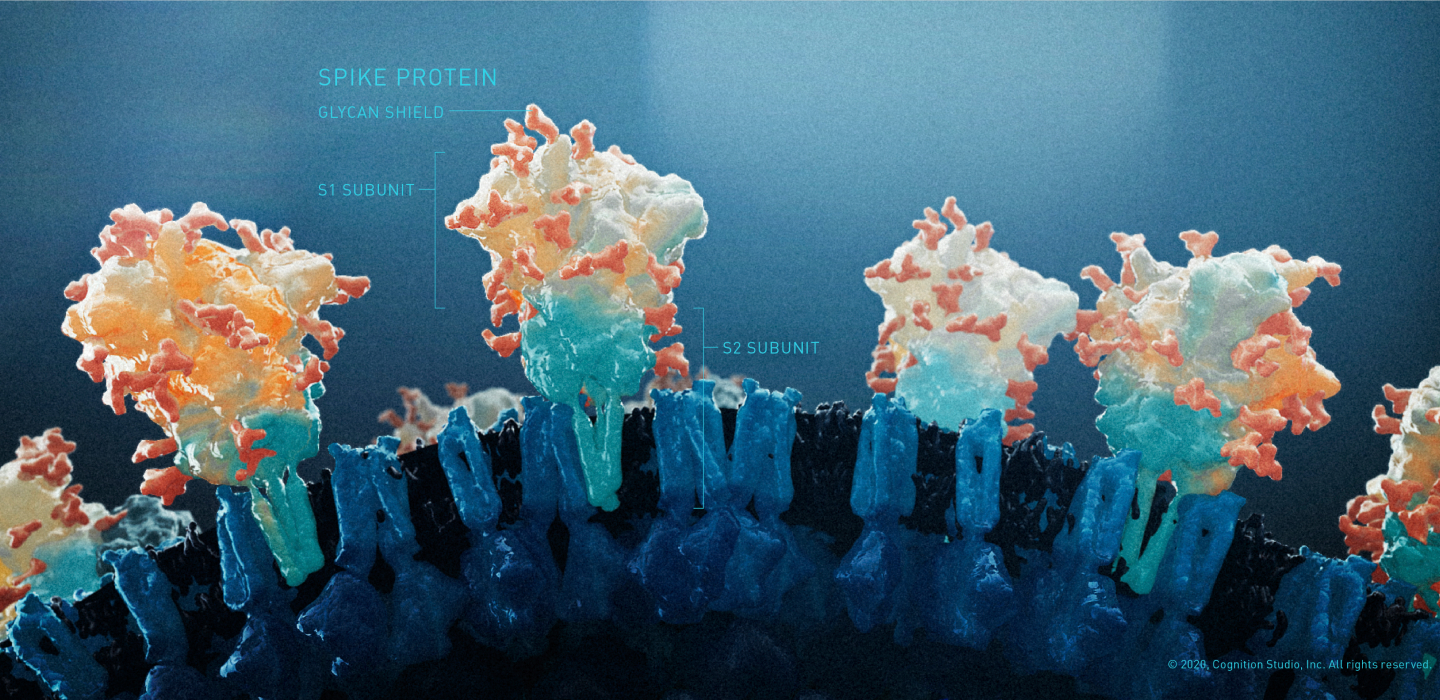
There was a growing body of evidence that suggested that fully vaccinated people were less likely to have an asymptomatic infection and potentially less likely to transmit SARS-CoV-2. In addition, preliminary studies showed some protection against emerging variants of concern (VOC), most notably the Delta Variant (B.1.617, originally identified in India), which showed increased transmissibility and reduced neutralizing antibody response and was the main variant circulating in the United States at the time;24 and the Omicron Variant (B.1.1.529, originally identified in South-Africa), which was believed to spread more easily than the original SARS-CoV-2 virus.25
Preventative measures such as mask use and social distancing continued to be important and were still recommended in public indoor spaces for both vaccinated and unvaccinated individuals.20